Introduction
Sports competition is an ideal context within which to study stress, as athletes are exposed to a number of factors that can trigger different physiological and behavioural responses. The hypothalamic-pituitary-adrenal (HPA) axis is activated by these stressful stimuli provoking a release of “stress hormones” into the bloodstream preparing the body to address these compromising situations (Iellamo et al., 2003). In this sense, the HPA axis can be stimulated in anticipation of a wide range of psychological stressors (Gaab et al., 2005) among which pre-competitive anxiety is the most common one.
Several hormones such us adrenocorticotropin (ACTH) and cortisol are representative of the HPA axis, although endorphins (END) and prolactin (PRL) are also considered stress hormones because they are secreted by the anterior pituitary gland in response to different stressors (Hackney et al., 1995). Indeed, a number of studies have been performed evaluating changes in the circulating levels of cortisol in athletes in response to psychological stress related to sports competition (Cintineo and Arent, 2019; Park et al., 2020; Slimani et al., 2018; van Paridon et al., 2017). However, the response of pituitary hormones could inform about the stress (physiological and psychological) experienced by athletes in both training and competitive efforts (Carrasco et al., 2007). Although reported data are limited, studies such as the one developed by Choi et al. (2013) observed an increase in serum END concentration as anticipatory stress response to sport competition. On the other hand, contradicting previously published results, Dmitrasinovic et al. (2016) did not find changes in ACTH concentrations measured 24 h before competition in ten amateur rugby players. In the same line, Suay et al. (1999) reported no differences in PRL levels when 28 judo fighters were evaluated before competition and training sessions. Nevertheless, and contrary to those findings, Carrasco et al. (2007) found a small anticipatory PRL response in swimmers just before competitiion.
Regarding physiological (somatic) stress, numerous investigations have evaluated the reactivity of the HPA axis through the responses of END, ACTH and PRL to exercise. In this sense, it has been demonstrated that these hormones are secreted into the bloodstream during both aerobic (Suzuki et al., 2000) and anaerobic exercise (Carrasco et al., 2007; Schulz et al., 2000). In any case, it seems that intensities around the anaerobic threshold are required to induce the release of END, ACTH and PRL into the circulation (De Vries et al., 2000).
Nevertheless, as previously mentioned, a measure of whole stress experienced by athletes during training and, especially, in competitive environments could be determined by measuring circulating levels of these hormones. This is important since a great number of children and young athletes are involved in competitive sports programs that could lead to severe stress and training overload states. At this point, and referring to the transactional perspective of stress and the cognitive-motivational-relational theory of emotions (Lazarus, 2000), athletes may experience a number of emotions, including anxiety, which can be a key predictor of the quality and duration of their sports careers (Scanlan et al., 2005). Taken into account that emotions are generally more intense during competitive than during non-competitive conditions (i.e., training sessions) (Lazarus, 2000), and that physiological demands are usually higher (maximal) in competitive efforts, a comparison of stress hormones concentrations in athletes under these situations could be useful to detect stress-related disorders and to prevent their harmful effects. Under these considerations, the purposes of this study were a) to define and to compare the END, ACTH and PRL responses to training and competition; b) to determine the contribution of psychological and physical stress dimensions to these responses, and c) to check the possible relationship between state-anxiety (SAn) levels and END, ACTH, and PRL responses to competitive and non-competitive sports practice.
Methods
Participants
Twelve young national-level male swimmers (age: 18.6 ± 0.8 years; body height: 1.75 ± 0.18 m; body mass: 65.11 ± 2.14 kg; BMI: 21.28 ± 0.66 kg/m2; body fat: 10.75 ± 1.00%), all of them specialists at distances of 100 and 200 m with an experience of at least 6 years in competitive swimming (15.5 ± 1.9 competitions per year) participated in this investigation. Informed written consent was obtained from all participants, and the study was approved by the local ethics committee.
Procedures
Participants were evaluated at four separate occasions, one week apart: baseline conditions (t0), before a regular training session (t1), before official swimming competition (t2), and after competitive effort (t3). For the evaluation at t0, participants were asked to abstain from strenuous physical activity for at least 24 h and to fast for 8 h before testing. Upon arrival to the laboratory (between 8:30 and 9:30 am), anthropometrical and body composition variables: height, body mass, and body mass index (BMI) were assessed. A blood sample (10 ml) was obtained from their antecubital vein and they also were evaluated for state and trait anxiety. At t1, participants were tested in the pool of their own swimming team maintaining the same conditions as indicated for t0. After blood samples were collected from participants (10 ml), the state anxiety levels were assessed just before they took part in a regular training session. Testing under competitive conditions was conducted at the same swimming pool with similar air and water temperature (27.5⍛ C and 29.4 ⍛C, respectively) and relative air humidity (58%) to those registered at t1. Once at the pool (8:00 am), participants rested for 10 min after which state anxiety was evaluated and a blood sample (10 ml) was obtained from swimmers (t2). Then, participants performed a 20 min standard warm-up (low-intensity swimming). Immediately after the warm-up, swimmers took part in their respective 100 m freestyle heats (between 8:40 and 9:40 am). The time to cover this distance was assessed by two official timekeepers and the mean value was used as an official result. The first 2 min after competitive effort (t3) were dedicated to obtain a new blood sample (10 ml) and to evaluate the state anxiety levels.
Hormonal and biochemical assessments. Each blood sample was divided into two aliquots of 5 ml (EDTA K2 tubes with 50 μl of aprotinin: SIGMA A6279). One of them was destined to haematological analysis (Coulter JT3 photocolorimeter), whereas plasma was obtained by centrifugation (3000 rpm, 15 min) from the remaining aliquot and stored at -80 ⍛C until its analysis. Plasma END, ACTH and PRL concentrations in t0, t1, t2, and t3 were measured by radioimmunoassay technique using commercial kits (END: Nichols, I.D. Intra-assay variation: 4.1%; inter-assay variation: 7.7%; ACTH: Nichols, I.D. Intra-assay variation: 1.4%; inter-assay variation: 4.6%; PRL: Radim-Ibérica. Intra-assay variation: 4%; inter-assay variation: 6%). Changes in END, ACTH, and PRL plasma levels through the four assessment points were considered as delta (Δ) values.
On the other hand, haemoglobin concentration and haematocrit value were assessed in order to check and correct the possible changes in plasma volume (PV) that could alter the results of the biochemical analysis in t3 (Dill and Costill, 1974).
State and trait anxiety assessment. The State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1994) was used to assess the anxiety proneness (trait anxiety) and the situational anxiety (state anxiety) of participants. Both state (SAn) and trait anxiety (TAn) scales were administered at t0 whereas only the state anxiety scale was applied at t1, t2, and t3. In any case, and in order to avoid the influence of discomfort related to awaiting venipuncture, the STAI questionnaire was completed by participants before blood samples were taken. Changes in SAn levels between the four-time points were also expressed as ΔSAn values.
Statistical Analysis
Statistical analysis was performed with SPSS v24.0 (SPSS Inc, Chicago, IL). Data are expressed as mean ± standard deviation (SD). The Kolmogorov-Smirnov test was conducted to check the normality of variables. Repeated measures ANOVA was applied to normally distributed data examining also skewness and sphericity; if assumptions of sphericity were violated the Huyn-Felt Epsilon was used. Friedman and Wilcoxon signed rank tests were performed to contrast the effect of within-subject factor (time points) of non-parametric variables. Effect sizes were measured using r (r = Z/√N) for non-normally distributed data, partial η2 for ANOVA analysis results and the benchmarks provided by Cohen (1988). Furthermore, Pearson and Spearman correlation coefficients (r and ρ, respectively) were calculated to determine the relationship between variables. In all cases, statistical significance was established at an alpha level of .05.
Results
Regarding anxiety, the mean TAn score measured at t0 was 21.8 ± 10.9 (CI: 95%: 15.19 – 28.34); on the other hand, SAn scores, which were measured at all time-points (t0-t3), are shown in Figure 1. The Friedman test showed statistical differences when intra-subject analysis for SAn was performed (p = .024) and the Wilcoxon signed range test demonstrated significant differences in SAn between t0 and t2 (18.5 ± 10.1 vs. 24.5 ± 11.2, respectively; p = .004, r = .806), and between t1 (18.7 ± 10.1) and t2 (p = .005, r = .785). On the other hand, there were no significant differences in SAn scores between t0 and t1 (p = .180) and between t0 and t3 (22.7 ± 11.5; p = .479). Considering ΔSAn data, the results confirmed the remarkable rise in SAn observed at t2, whereas ΔSAn values assessed from t0 to t3 did not show statistical significance when they were compared to those obtained from t0 to t1; however, SAn differences measured between t3 and t0 (Δt3-t0) and between t3 and t2 (Δt3-t2) showed statistical significance (Table 1), especially if one took into account that values assessed at t3 were lower than those registered at t2.
Figure 1
Raw data of state anxiety (SAn) self-reported by swimmers at the four assessment time-points considered. t0 = baseline conditions; t1= before a regular training session; t2 = before real swimming competition; t3 = immediately after the competitive effort. **p < .01; r = effect size.
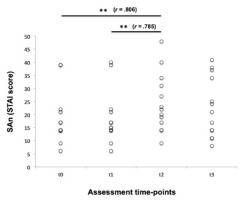
Table 1
Differences between delta (Δ) mean scores for SAn, END, ACTH, and PRL
[i] Values are expressed as mean ± SD (CI 95%). SAn = state anxiety; END = endorphins; ACTH = adrenocorticotropin; PRL = prolactin. Z-value = Wilcoxon signed-rank test Z statistic; F-value = repeated measures ANOVA F statistic; pη2 = partial eta squared; r = effect size for non-normally distributed data; **p < .01 = significant differences between t1-t0 and t2-t0; ††p < .01 and †††p < .001 = significant differences between t2-t0 and t3-t2; ‡p < .05 and ‡‡p < .01 = significant difference between t3-t0 and t3-t2;
§§p < .01 and §§§p < .001 = significant differences between t2-t0 and t3-t0.
Regarding biochemical data and taking into account changes in PV observed between t2 and t3, it is important to note that plasma concentrations assessed at t3 were individually corrected since PV decreased over 10% (11.42 ± 4.62) after competitive effort (61.47 ± 7.14 s of maximal swimming). Focusing on END analysis [(F(3,36) = 32.96; p < .001)], plasma levels of this stress hormone were very similar at t0 and t1 (36.3 ± 10.5 and 33.0 ± 10.5 pg/ml, respectively; Figure 2A). On the other hand, a remarkable END response at t2 was measured, reaching mean values of 51.8 ± 11.5 pg/ml which were significantly higher than those registered at t0 and t1 (p = .011 and p = .001, respectively). However, the greatest release of END occurred at t3 (128.6 ± 65.4 pg/ml) showing statistical differences with values measured at t0 (p < .001), t1 (p < .001), and t2 (p < .005). Moreover, effect size calculations reported a partial η2 value of 0.733 for these within-subject contrasts (large effect size). As it can be seen in Table 1, data confirmed a slight decrease of END levels at t1 and the largest responses of END at t2 and mainly at t3. At this point, it is important to note that significant differences were found when Δt2-t0 and Δt1-t0 were compared. Statistical significance was also reached after Δt3-t2 and Δt2-t0 were contrasted. Considering t3 as the assessment point at which END showed the highest values, it seems obvious that the comparisons of Δt3-t0 to Δt3-t2 and Δt3-t0 to Δt2-t0 were statistically significant (p = .005 and p = .001, respectively).
Figure 2
A) Endorphins (END), B) adrenocorticotropin (ACTH), and C) prolactin (PRL) plasma levels measured at the four assessment time-points considered. t0 = basal conditions; t1 = before regular training session; t2 = before real swimming competition; t3 = immediately after the competitive effort. *p < .05; **p < .01; ***p < .001; pη2 = partial eta squared for within-subject contrasts (ANOVA); r = effect size for non-normally distributed data.
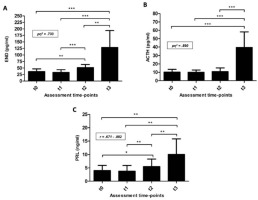
On the other hand, the pattern of ACTH responses was, in part, similar to END. In this way, and compared with t0, a small decrease in ACTH plasma levels was found at t1 (10.15 ± 3.37 and 9.95 ± 2.79 pg/ml, respectively) (Figure 2B). However, unlike the END responses, increases in ACTH levels at t2 (10.88 ± 4.30 pg/ml) did not show statistical differences with those abovementioned. After competitive swimming effort (t3) a huge response of ACTH was observed (39.37 ± 18.73 pg/ml) so this increase was, approximately, four-fold higher than those observed at t0 and t1 [(F(3,36) = 39.84; p < .001)]. Additionally, effect size calculations showed a partial η2 value of 0.890 for these within-subject contrasts (large effect size). These results are consistent with data comparisons between assessment time-points (Table 1); indeed, taking the t1-t0 comparison as a reference, the greatest increments of ACTH were observed in Δt3-t0 and Δt3-t2 (p = .001 in both cases).
Regarding changes in PRL plasma levels, and as it happened with END and ACTH, there was no statistical difference between t0 and t1; certainly, a slight decrease was observed (4.02 ± 1.93 and 3.75 ± 2.17 ng/ml for t0 and t1, respectively). However, a small but significant increase was found at t2 (5.52 ± 2.80 ng/ml; p < .01, r = .765), although the greatest values were registered at t3 (10.07 ± 5.75 ng/ml; p < .01, r = .882) (Figure 2C). Contrasting analysis of PRL mean delta scores showed the same trend as END ones (Table 1), since comparisons of Δt3-t0 to Δt3-t2 and Δt3-t0 to Δt2-t0 were statistically significant (p < .05 and p < .01, respectively).
Correlation analysis revealed interesting relationships between variables. TAn was positively correlated with SAn measured at t2, whereas this SAn at t2 showed strong links with SAn at t0 (ρ = .824; p = .001) and t1 (ρ = .836; p < .001). Although no relationships were assessed between SAn and stress hormones levels measured at t0, t1, and t2, significant correlations were found at t3 (ρ = .626, p = .022 for SAn and END; ρ = .648, p = .017 for SAn and ACTH, and ρ = .573, p = .041 for SAn and PRL). Once stress hormone values were contrasted among themselves, we found significant relationships. In this way, END levels measured at t2 were correlated with those measured for ACTH (r = .647, p = .017), and PRL (ρ = .574, p = .04) at the same assessment time-point. Moreover, END showed significant correlation with ACTH at t3 (r = .725, p = .005). ACTH concentrations at t0 were correlated with those found for this stress hormone at t2 (r = .791, p = .001), and t3 (r = .610, p = .027). Additionally, ACTH levels at t0 showed significant correlation with PRL at the same assessment time-point (ρ = .698, p = .008). Furthermore, PRL levels measured at t2 correlated significantly with those measured at t3 (ρ = .746, p = .003).
Discussion
The main aim of this study was to compare the response of several stress hormones to different sports situations (competition and regular training sessions) evaluating the relationship with SAn expressed by youth swimmers under both conditions. Moreover, this study highlighted the connection between psychological and physical responses to stress induced by a competitive sports event, taking training conditions as references for evaluating their magnitudes.
Baseline levels of END, ACTH and PRL were in consonance (although slightly lower) with those previously found in the target population (Mielgo-Ayuso et al., 2017; Sönksen et al., 2018; Suzuki et al., 2000). Although in the present study we did not consider a control group, the similarity of these results with the reference data does not allow to establish a clear exercise-adaptive effect on the baseline levels of these hormones (Duclos et al., 2001).
As it could be expected, no changes were observed in END, ACTH, and PRL levels when swimmers were evaluated in pre-training condition; indeed, we observed a slight decrease in these plasma concentrations. This could be due to the fact that the laboratory environment acted as a stressor agent (Moya-Albiol and Salvador, 2001). Moreover, the baseline condition was the first assessment time point and the expectations of participants involved in the investigation could generate some anticipatory anxiety.
At this point, and according to the lack of stress response, we observed low TAn (t0) and SAn scores (t0 and t1) being in the same range of those corresponding to the age-matched Spanish population (Guillén-Riquelme and Buela-Casal, 2011). In spite of this coincidence, END, ACTH and PRL plasma concentrations were not significantly correlated to TAn or SAn scores.
On the other hand, significant increases in END and PRL plasma concentrations were found at t2, indicating that psychological stress and/or anxiety experienced by swimmers before participating in the official competition activated the HPA axis and, consequently, increased these hormone levels. In fact, we observed a remarkable rise in SAn scores from t1 to t2, and especially from t0 to t2, which is in line with the results of previous studies such as those conducted by Fernandez-Fernandez et al. (2015) and Souza et al. (2018). Although those authors investigated psychophysiological stress responses using different questionnaires and HPA hormones to those assessed here, the response to stress (anxiety) perceived before official competitions (tennis match, Jjiu-Jitsu combat, running and canoeing races) provoked a significant increase in salivary glucocorticoids compared to values obtained before training sessions. Moreover, it is important to highlight the PRL response to psychological stress since previous studies did not find increases in plasma PRL levels as a result of precompetitive anxiety (Suay et al., 1999). According to these authors, and unlike END and ACTH, PRL does not seem responsive to psychological stress. By contrast, we reported a similar pattern in END and PRL responses to the precompetitive situation (although no significant correlations between these hormones were observed).
However, in our study, the response of ACTH to the psychological stress-related competition was slight, showing no statistical differences with baseline (t0) and pre-training session (t1) conditions. These results are consistent with those reported by Dmitrasinovic et al. (2016) who also found no differences in ACTH levels measured in both baseline and pre-competitive status in a group of amateur rugby players. It is possible that pre-competitive anxiety experienced by our swimmers was not a sufficient stimulus to induce a sensitive ACTH response. At this point it is important to note that blood samples at t2 were taken 20 min before the start of the competition; although SAn increased significantly from t0 (and t1), plasma ACTH concentrations were unchanged so we can hypothesize that stress hormones react differently depending on the intensity of the psychological stressor. Another possible explanation can be found in the shortened half-life of ACTH (of around 3 min) that may account for decreased availability of this stress hormone. However, and paradoxically, ACTH plasma levels showed a significant relationship with END concentration at t2. Taking into account that END and ACTH share the same precursor (proopiomelanocortin; POMC) and the huge increase in END at t2 which could be due to the psychological stress, it could be hypothesized that pre-competitive anxiety produces different effects on the stress hormone responses.
Just after the competitive swimming effort, we observed significant increases in END, ACTH and PRL plasma levels. Although swimmers performed a brief and maximal exercise, a remarkable END release was observed. These results are in agreement with those found in previous studies in which a single bout of intense exercise was evaluated. In this line, Schulz et al. (2000) reported increases of 10 times resting levels after a high-intensity exercise which included 1 min of maximal rowing. In our investigation, the END plasma levels assessed at t3 were approximately 250% higher than those measured at t0. This increase in plasma END concentration was associated with the HPA axis reactivity to psychological (anxiety) and physical (exercise) stress. However, if plasma END dynamics through the four assessment time-points are considered (Table 1), it is easy to appreciate different END response magnitudes depending on the prevalence of mental or physical stress. In this sense, the most remarkable increases of plasma END levels occurred when the interval t3 - t0 was established. This seems to indicate that physical stress is a more powerful stimulus than psychological stress, especially if one takes into account that HPA axis activation is required to ensure blood glucose homeostasis by stimulating gluconeogenesis (van Paridon et al., 2017). In fact, and as it happened with END response, plasma ACTH and PRL concentrations reached their maximum values at t3 (increases of 287% and 150%, respectively, in relation to baseline conditions). Moreover, our results are in consonance with those previously reported by Pompe et al. (2001), who found similar responses of ACTH and PRL after high-intensity exercises, but contrary to those described by Choi et al. (2013) and Bosco et al. (1996) who did not find significant increases in END and PRL levels after both kumdo competition and a single bout of anaerobic exercise, respectively. Although ACTH and PRL are normally used as biomarkers for overload and/or overtraining detection in athletes (van Paridon et al., 2017; Walker et al., 2019, 2020), the psychophysiological role of these hormones during exercise does not seem to be sufficiently defined. Thus, further investigation focused on PRL responses to exercise-induced stress is important since PRL could also be involved in reproductive cycle dysfunction and contribute to the female athlete triad (Walker et al., 2019, 2020).
Despite this, SAn decreased at this time-point assessment compared to t2, whilst being slightly higher than the values registered at t0. Taken into account that swimmers did not have immediate information about their classifications in competition, our results are according to those reported previously in the literature. Moreover, and although it is not possible to differentiate between the exact contribution of psychological and physiological stress to the whole stress response at t3, it could be hypothesized that physical stress has a greater effect on these stress hormones than the mental one.
On the other hand, and with the exception of the relationships defined at t3, no correlations were found between stress hormones and SAn. This lack of correlation could be due to the use of a non-specific tool to assess the anxiety levels. SAn was evaluated using the state scale of the STAI. Unlike other investigations in which specific questionnaires were applied (mainly CSAI-2), we chose this scale because besides the pre-competitive condition we measured the anxiety levels in three other situations: baseline, pre-training session, and post-competition (essentially, the CSAI-2 is focused on the assessment of pre-competition anxiety). Moreover, several items of the state-anxiety scale of the STAI have served as a reference in the design of different questionnaires, including the CSAI and CSAI-2 (Arruza et al., 2010). Anyway, another explanation for this lack of association may be related to the influence of some psychological vari¬ables, such as motivation, mental toughness, self-efficacy and self-confidence which could play a key role as mediator variables between hormonal response and competitive anxiety (Slimani et al., 2018).
Although the assessment of athlete’s responses to stress during real sports competition (and their comparison with those provoked by a regular training session) can be understood as the key contribution of the present study, several limitations must be considered. First, the sample size was relatively small, which did not prevent from detection of statistically significant results regarding stress hormone responses, but may have limited the relationship between variables. However, it is necessary to highlight the difficulty found in the use of invasive techniques (i.e., blood samples collection) on athletes who were competing in a major national competition. Second, and taking into account the above mentioned, blood collection and SAn assessment at t2 had to be performed 20-min before the swimmers’ participation in competition. While the stress responses were significant, it is possible that those found just prior to competition would be more relevant than those reported here. Lastly, large interindividual differences in the responses of stress hormones can lead to compromise their relationships with other variables such as psychological or cognitive variables. As it has been described before, both mental and physical stress could induce distinct emotional and physiological responses in athletes during sports competition (Souza et al., 2018).
We can conclude that pre-competitive anxiety induces an important response of stress hormones (especially END and PRL) in youth swimmers. Attending to those values, the psychophysiological load of regular training sessions can be assessed using individual analyses and comparisons. Moreover, and taking into account the magnitude of stress hormone responses in the four assessment time-points analyzed, it seems that both mental and physiological stress boost the release of END, ACTH and PRL during sports competition. Regarding the third objective, and considering the abovementioned limitations, a lack of correlation between END, ACTH and PRL plasma concentrations and SAn levels was registered, thus new studies with larger sample sizes are needed to determine the relationship between neuroendocrine and cognitive reactivity to stress.


