Introduction
The structure of muscle activity and co-activation during technical actions in wheelchair fencing has not been the subject of research. Reports on muscle activation and co-activation in other combat sports, e.g., karate, were published by Quinzi et al. (2015) and Sbriccoli et al. (2010). Technical actions in wheelchair fencing, such as direct and compound attacks, ripostes and counter-ripostes are characterized by their remarkable dynamics based on anaerobic processes. It is therefore justified to claim that due to overlearning, higher levels of activity of antagonistic and agonistic muscles are reached in wheelchair fencing training, which should be reflected in higher muscle co-activation indices (CI). A factor reinforcing the co-activation process is the anticipatory activation of postural muscles, which contributes to the effectiveness of the direct fencing thrust to a visual stimulus.
The recorded EMG signal structure during fencing attacks is a key factor in assessing the correct movement pattern, mainly the sequence of activation of muscle groups in response to a given stimulus. Wheelchair fencers under study were categorized into two disability-level groups:
Group A - amputees or individuals with mild paralysis of the lower limbs; and Group B athletes with spinal cord injuries and paraplegia and paresis of the arms. The movement patterns in Group B fencers were found to be most often modified in subsequent actions, along with varying bioelectrical muscle tensions. In contrast, fencers from Group A generated relatively low tension levels with a similar activation pattern of selected muscles. According to the recorded pattern of muscle activation during a direct thrust to a visual stimulus (a movement of the coach's blade), the fencer’s muscles of the upper limb, abdomen and back are the first to be activated, constituting a specific postural system (Borysiuk et al., 2020a; Farrokhian et al., 2021; Ying-ki et al., 2010, 2013).
The recording of bioelectrical activity of muscles can demonstrate significant differences among athletes, even in repetitive motor activities. These differences usually concern EMG signal values, times of muscle activation, fatigue processes, but also the concurrent activities of muscle pairs, i.e., muscle co-activation, when agonist and antagonist muscles simultaneously co-contract within a joint (Klass et al., 2007; Le et al., 2017; Lundy-Ekman, 2013; Riemann and Lephart, 2002). Co-activation refers to two muscles contracting at the same time, expressed by the percent of movement in which both muscles are simultaneously active. Most commonly, muscle co-activation can be calculated from pre-recorded muscle contractions (activity) using surface EMG. However, a decrease in the co-activation index, calculated as antagonistic muscle activity divided by agonistic muscle activity, indicates a higher level of agonistic activity or a lower level of antagonistic activity resisting the movement (Barrata et al., 1988; Farrokhian et al., 2021; Hamada et al., 2000; Kellis et al., 2003; Sale, 2004). The co-activation mechanism is important for the stabilization of given joints, and it promotes motor control of particular actions (Aagaard et al., 2000; Bazzucchi et al., 2004, 2006; Sbriccoli et al., 2010).
The main research tool in the present study was surface electromyography (sEMG) used to determine the onset of activation of particular muscles and co-activation of muscle pairs: TRI-BC, ECR-FCR, LD RT-EOA RT, LD LT-EOA LT. The following assumptions were made:
the muscles of the abdomen and the back play a significant role in the muscle activation structure, and they are activated earlier, i.e., at the same time as the activation of the sword arm extensor (TRI);
longer activation of most muscles in Group B fencers is expected since these muscles play a stabilizing role, protecting against injuries;
with regard to co-activation indices, due to similar patterns of recruitment of fast motor units, no significant differences are expected between wheelchair fencers from Groups A and B.
Methods
Participants
Sixteen wheelchair fencers of the Polish National Paralympic Team (7 in Group A, and 9 in Group B) took part in the study. The fencers’ mean characteristics were:
Group A: age (years) – 33.58(±6.26), body height (m) – 1.74(±0.08), body mass (kg) – 69.01(±8.92), training experience (years) – 15.28(±6.31);
Group B: age (years) – 31.33(±9.68), body height (m) – 1.64(±0.10), body mass (kg) – 58.66(±10.29), training experience (years) – 7.01(±2.41).
All the fencers were right-handed, represented a high international sporting level, and included multiple medal winners of the Paralympic Games.
Measures
The study was approved by the Bioethics Committee of the Opole Medical Chamber (Resolution No. 237 of 13 December 2016), and it was in accordance with the Declaration of Helsinki guidelines regarding the conduct of clinical trials on humans.
The study was conducted using a 16-channel EMG system (Noraxon, DTS, Desktop Direct Transmission System, Scottsdale, Arizona, USA) with 16-bit sampling accuracy at 1500 Hz. The MyoResearch XP Master Edition for DTS Noraxon system was used for data analysis. To synchronize the EMG system, a wireless unit (a 3-axis wireless DTS 3D accelerometer sensor with the nominal output range of +/-6g, sensitivity of +/-0.67V/g, and bandwidth of 5Hz–1.8kHz) was used to transmit the EMG signal directly to the PC. The EMG signals were subjected to data rectification.
The sequence of bioelectrical muscle activity was determined by manually selecting the onset and offset of the raw recording of a particular muscle activity while observing the EMG signal waveform (Crotty et al., 2021).
The testing procedure followed the SENIAM project guidelines (Hermens et al., 2000). The sets of fencing actions were divided into three attempts in a single set of direct thrusts to a visual stimulus.
Muscle activity time was determined on the basis of three attempts performed in one set. The attempts were divided into single sequences. The activity time was calculated as the percent ratio of three attempts to the time of the whole activity.
Co-activation Index (CI)
To assess the relative level of co-activation (simultaneous contraction of both muscles) for the TRI-BC, ECR-FCR, LD RT-EDA RT or LD LT-EDA LT muscle pairs, the co-activation index (CI) was calculated following the methodology by Falconer and Winter (Falconer and Winter, 1985; Nagai et al., 2011) using the equation:
where: Iant - the area of total antagonist muscle activity expressed as:
where: t1-t2 - the time period during which EMGANT1 is lower than EMGANT2; t2-t3 - the time period during which EMGANT2 is lower than EMGANT1; Itotal - the integral of the sum of EMGANT1 and EMGANT2 during the execution of the task. Data were calculated using the following equation:
Design and Procedures
The tests were preceded by a 20-25-min individual warm-up which prepared fencers for the tests. During the tests EMG electrodes were placed on the fencer's body along nine channels: arm muscles (DEL, TRI and BC), forearm muscles (ECR, FCR), the external abdominal oblique muscles and the back muscle (EOA RT and LT, LD RT and LT).
Prior to the execution of the attempts, a fencing platform with the fencer's wheelchair was set up, and the distance from the tip of the fencer’s weapon to the coach's flexed arm was measured. The correct distance between the fencer and the coach was the distance between the end of the fencer's weapon and the coach's elbow (Borysiuk et al., 2020a). An accelerometer placed on the guard of the coach's weapon was used to indicate the fencer’s response to the coach's blade motion. The coach initiated three attempts of direct thrusts to a visual stimulus (coach's blade movement from parry quarte to parry sixte) in one set (Photo 1).
Statistical Analysis
The collected data were processed using Jamovi (R Core Team, 2021; Lenth, 2020). The study hypotheses were verified at the level of significance of p≤0.05 (Welch’s t-test). The normal distribution of analyzed statistical features was checked with the Shapiro-Wilk test.
Results
Figures 1a and 1b and Table 1 present the activity times from the three attempts in one set and the time of the whole muscle activity (0-7) to a visual stimulus. The values shown are taken from data of two representative fencers from Groups A and B.
Figures 1a, 1b
Activation times of particular muscles to a visual stimulus (3 attempts in 1 set) in wheelchair fencers from Group A (1a) and Group B (1b).
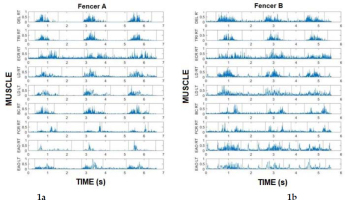
Table 1
Activation time of particular muscles to a visual stimulus (3 attempts in one set) in fencers from Groups A and B.
Table 1 shows the muscle activation times as a ratio (%) of three performed attempts to total activity time of a given muscle in one set. Group A fencers demonstrated shorter activation time in almost all muscles, except for the ECR (58.24), than Group B fencers.
Postural muscles (abdominal and back) were activated faster, which confirms the validity of the postural muscle activation sequence. This is due to the activation of antagonist muscles representing a centrally programmed anticipatory mechanism affecting the stabilization of technical actions.
Table 2 and Figures 2a-2d illustrate the co-activation values (in response to a visual stimulus) in three thrusting attempts in one set among the fencers from Groups A and B.
Figure 2a
Statistical analysis (Welch's t-test) of co-activation differences for forearm muscle pairs (ECR - FCR) activated to a visual stimulus in Groups A and B.
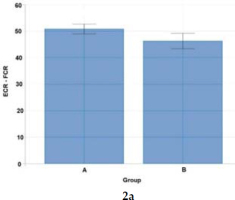
Figure 2b
Statistical analysis (Welch's t-test) of co-activation differences for upper limb muscle pairs (TRI - BC) activated to a visual stimulus in Groups A and B.
Notes: ECR - extensor carpi radialis longus, FCR - flexor carpi radialis, TRI – triceps brachii, BC – biceps brachii
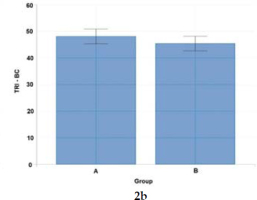
Figure 2c
Statistical analysis (Welch's t-test) of co-activation differences for right-side postural (abdominal and back) muscle pairs (LD RT - EOA RT) activated to a visual stimulus in Groups A and B.
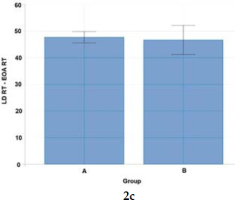
Figure 2d
Statistical analysis (Welch's t-test) of co-activation differences for left-side postural (abdominal and back) muscle pairs (LD LT - EOA LT) activated to a visual stimulus in Groups A and B.
Notes: LD RT – latissimus dorsi of the right or the left side, EOA RT - external oblique abdominal of the right or the left side
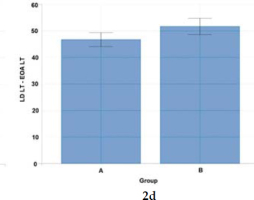
Table 2
Statistical analysis (Welch's t-test) for arm, forearm, abdominal and back muscle pairs activated to a visual stimulus in fencers from Groups A and B.
Data from Figures 2a and Table 2 indicate a high effect size [1.02] underlining the significance of forearm muscle pairs (ECR – FRC) in the movement pattern structure in wheelchair fencers from Group A.
The effect size was high [0.94] reflecting the significance of left-side pairs of muscles of the back and the abdomen (LD LT-EOA LT) for Group B fencers. The study confirmed the significance of postural muscles in the movement pattern structure for right-handed wheelchair fencers from Group B (Figure 2d, Table 2).
Discussion
EMG studies of athletes with physical disabilities in fencing are gaining increasingly more recognition in the world of sport. As a result also the awareness of the role of competitive sports in improving the health and quality of life of people with disabilities grows within the general population. Thanks to the use of EMG, a number of interesting research prospects have appeared in the area of Paralympic disciplines, for example, the bench press. Gołaś et al. (2017) demonstrated that disabled lifters performed each task with the complete involvement of the central nervous system in the control and regulation of motor activities (mainly complex tasks with participants’ awareness during the movement). The CNS is mainly responsible for the activation of particular muscle groups according to a predetermined sequence known as the movement pattern, including biofeedback (feedback during movement) (Nichols et al., 1999). The mentioned authors demonstrated increased synchronization of motor units together with a greater involvement of particular groups of muscles of the upper limbs during the bench press exercise. All changes in the activation of particular muscles should be analyzed in accordance with the adopted structure of performed movement, both in able-bodied and disabled athletes.
The research of movement patterns among wheelchair fencers constitutes an innovative approach addressing the current needs of high-performance athletes. The present study is the first one in literature to use the EMG to determine muscle co-activation and muscle activity time in Paralympic wheelchair fencers. Our results revealed that the co-activation index between 48 and 51% ensures full coordination of movements and, as a significant indicator of movement synergy, a high level of motor coordination, including postural balance.
Reports of muscle co-activation in fencers by, among others, Pensini et al. (2002), indicate a significant positive effect of eccentric training on the range of motion during the lunge (by increasing agonist work during voluntary activation and decreasing antagonist co-activation). Di Cagno et al. (2020) as well as Gutierrez-Davila et al. (2014) reported similar findings in their studies on eccentric work during the lunge and the advance-advance lunge.
Consistent with previous studies of wheelchair fencers' movement patterns in response to visual stimuli (Borysiuk et al., 2020a, 2020b), the importance of postural muscles (abdominal and back) should be emphasized in fencing training. The activity of these muscles constitutes a set of postural stabilization factors. This mechanism determines the specificity of neuromuscular control in wheelchair fencers (Borysiuk et al., 2018; Quinzi et al., 2013, 2014; Sbriccoli et al., 2010). A fencing attack to the opponent's trunk requires decisive and maximum speed execution. A wheelchair fencing duel is characterized by particular dynamics, and it makes the fencers simultaneously engage multiple pairs of muscles, such as muscles of the upper limb, abdomen and back. Wheelchair fencing is classified as a combat sport in which coordination predispositions prevail together with motor abilities such as endurance, and movement characteristics such as explosiveness (Borysiuk et al., 2020a). Moreover, wheelchair fencers are also characterized by their high precision of technical actions combined with the coordinated activity of the muscles of the sword arm and with core stability. These characteristics mean that studies of co-activation strategies (Quinzi et al., 2015) can yield valuable information about the work of key opposing muscles.
Studies of muscle co-activation show that wheelchair fencers are characterized by varying patterns of muscle co-activation indices expressed in percent. In view of actions to a visual stimulus (thrusts to the trunk in response to the coach's blade motion), wheelchair fencers from Group A demonstrated higher co-activation of the right-side postural muscles of the back and the abdomen (50.94), and of upper limb muscle pairs: ECR-FCR (50.75) and TRI-BC (47.99). In contrast, Group B fencers reported higher co-activation only for left-side postural muscle pairs (50.54). As confirmed by other authors, higher antagonist muscle activity levels were also observed in elite athletes (karate competitors) as opposed to novice athletes (Purves, 2012; Quinzi et al., 2015).
In offensive fencing actions to a visual stimulus the muscles with higher differences in the activity of given pairs, as confirmed in the present study, included the upper limb muscles in Group A fencers: ECR-FCR [Cohen's d = 1.02], and the postural muscles on the left side: LD LT-EOA LT in Group B fencers [Cohen's d = 0.94]. This demonstrates the high specialization of the leading muscles in response to a given visual stimulus with regard to the quality of wheelchair fencing technique. The pattern of wheelchair fencers’ psycho-motor responses in earlier studies (Borysiuk et al., 2020a, 2020b) corresponds with the results of analysis of co-activation of selected muscle pairs in the present study. It can be assumed that during long-term learning of the technique by wheelchair fencers, due to motor control, some kind of "re-modelling" takes place resulting in an increase in the co-activation indices of postural muscles and forearm muscles determining the effectiveness of target hitting.
Based on the APA phenomenon (Borysiuk et al., 2020a), the activation of postural muscles determines the effectiveness of fencing techniques in able-bodied fencers and fencers with disabilities, which demonstrates the similarity of movement patterns in both groups. On the other hand, the activation of the left latissimus dorsi has a supporting function for the trunk, but not only because it is a component of the movement pattern which determines the speed of action in the context of% MVC EMG (high values).
The analysis of muscle activation time as a percent ratio of three performed attempts in a set confirmed the study assumptions. It turned out that fencers from Group A displayed faster activation in almost all muscles except for the ECR (58.24) compared to fencers from Group B. It can be said that postural muscles (abdominal and back) are activated faster, which confirms the validity of the sequence of postural muscle activation consistent with the movement pattern of wheelchair fencers (Borysiuk et al., 2020a). This is due to the activation of antagonist muscles representing a centrally programmed anticipatory mechanism affecting the stabilization of technical actions. On this basis, a general assumption that there is a longer activation time of most of the muscles in Group B fencers in intergroup comparisons can be accepted. These muscles play a stabilizing function protecting against injury and trauma. Finally, it can be assumed that the studied Paralympic wheelchair fencers representing two different disability-level groups demonstrated the desired standard co-activation index (CI) of their key muscles, within the approximate range of 4851%. For future studies and generalization of results, however, the sample size should be increased.
Practical implications
Although selected components of wheelchair fencing technique were assessed in previous research, the present study is the first to analyze the movement pattern described by the complex reaction time representing muscle activation and the level of bioelectric signals expressed with EMG. This new approach emphasizes the significant role of the work of postural muscles (back and abdominal) in training of offensive actions in wheelchair fencing, which mostly involves individual sessions (practical lessons) with coaches and free duels with teammates. In view of the above findings, it is recommended that postural muscles exercises be included in explosive strength training in wheelchair fencing. The activation of additional motor units should contribute to greater coordination and enhance movement speed in fencers.
Conclusions
Group A fencers displayed higher co-activation levels in postural muscles: back and abdominal muscles on the right side (50.94); and the upper limb muscle pairs: ECR-FCR (50.75) and TRI-BC (47.99). Group B fencers had higher co-activation values only for postural muscle pairs on the left side (50.54). This indicates that the opposite side to the attacking arm constitutes the postural basis for offensive actions of fencers with more severe levels of disability.
Fencers from Group A had a shorter activation time in all tested muscles, with the exception of the ECR (58.24), compared to fencers from Group B. This confirms the activation of antagonist muscles representing a centrally programmed anticipatory mechanism stabilizing technical actions as particularly intensified in Group A fencers.
The study results indicate that the standard co-activation index (CI) of key muscles involved in wheelchair fencing ranges from 48 to 51%.



