Introduction
During explosive, high-intensity exercise, performance success is determined by an athlete's ability to maintain a maximal effort ranging from few seconds to a few minutes in duration (Hanon et al., 2019). During high-intensity (HI) exercise, glycolysis contributes significantly to energy production, inducing disturbances in acid-base balance, particularly a decrease in muscle and blood pH and hyperlactatemia. Since metabolic acidosis limits the maintenance of physical activity and performance (Millet et al., 2004), increasing the buffer capacity could enhance HI anaerobic performance and reduce blood metabolic disturbances. This can be achieved either through training (Edge et al., 2006) or by the exogenous supply of buffering agents both acutely (Lancha Junior et al., 2015) and chronically (Chycki et al., 2018). One of the most popular buffering agents is sodium bicarbonate, which has been investigated in numerous studies (Grgic et al., 2021). The bicarbonate is typically provided via supplement with potential side effects, especially gastrointestinal distress. Nonetheless, it is important to note that some athletes may be unable to tolerate direct bicarbonate supplementation, as it can lead to gastrointestinal issues (McNaughton et al., 2016; Ragone et al., 2020). Certain coaches and athletes may not be inclined to embrace the concept of enhancing their buffering capacity through methods like bicarbonate supplementation. The enhancement of buffering capacity through a strategy perceived as more natural might receive approval within the sporting community. There is limited research examining supplementation through the regular consumption of naturally occurring water rich in bicarbonate ions (HCO3-). Chronic intake (1 week) of mineral water with a pH of around 6 and a concentration of HCO3- ions around 1000 mg/L has been shown to have either no effect (Chycki et al., 2017) or increase urinary pH (Brancaccio et al., 2012; Halz et al., 2022), after HI exercise. The lowest intake (1.5 L/day of water containing 981 mg/L of HCO3-) resulted in increased urine pH (Brancaccio et al., 2012), whereas the highest intake (4–4.5 L/day of water containing 1300 mg/L of HCO3-) did not (Chycki et al., 2017). An explanation could be the timing between the water ingestion and the urine collection. The urine pH was elevated when the water was ingested during the hours before urine collection (Brancaccio et al., 2012), suggesting that the observed effect might be due to acute administration rather than a chronic adaptation. Although those studies include exercise, performance was not measured, and blood markers of acid-base balance were poorly reported.
Consumption of water containing a high bicarbonate content (4368 mg/L) has favored a rapid restoration of acid-base balance after aerobic exercise and permitted greater residual strength following an isokinetic test (Rieux and Duvallet, 2000). In this study, bicarbonate-rich water was not ingested chronically as previously reported (Brancaccio et al., 2012; Chycki et al., 2017), but only on the day of testing (2 h before exercise until immediately before the last test). Altogether, the few data available highlighted the need for further studies regarding the effect of chronic bicarbonate-rich water intake on acid-base balance and anaerobic performance. It has been suggested that the ingestion of bicarbonate-rich water alongside an alkalizing diet may provide a novel nutritional strategy to influence acid-base balance (Limmer et al., 2018; Remer and Manz, 1995) and improve anaerobic performance (Limmer et al., 2018; Steffl et al., 2021). Combining bicarbonate-rich water with an alkalizing diet could enhance the benefits of increasing buffering capacity and potentially lead to improved performance. In contrast, when combined with an acidifying diet, bicarbonate-rich water may mitigate the negative effects of such a dietary pattern. This approach may offer a solution for athletes who prefer acid-forming foods like meat while minimizing the potential drawbacks of such a diet. As such, evaluating how the diet may modulate the effect of bicarbonate-rich water chronic ingestion on exercise performance warrants investigation.
The present study, therefore, aimed to investigate whether the consumption of bicarbonate-rich water combined with an alkalizing diet could provide an additional buffering effect, potentially delaying the effects of metabolic acidosis and improving performance. We also hypothesized that the consumption of bicarbonate-rich water could mitigate the acid load induced by the acidifying diet, particularly in terms of urinary and blood pH.
Methods
Participants
Twenty-four recreationally active men (29 ± 8.8 years, 1.79 ± 0.50 m, 81.8 ± 11.6 kg, VO2max 55.6 ± 9.0 ml/min/kg) volunteered to participate in this study. All participants were informed of the study protocol, their rights, and the associated risks of the intervenion before providing written informed consent. All procedures were approved by the committee CERSTAPS (approval No. 2022-A00644-39; approval date: 01 January 2020) and conducted in line with the Declaration of Helsinki (1964, revised 2001). Participants were excluded from the study if they could not follow dietary and hydration recommendations or upon the occurrence of any injury or unusual fatigue during the 4-week protocol that could cause a decrease in performance during the supramaximal test.
Experimental Design
Participants performed two pre-visits (pre- visit 1: consent signature, nutritional interview, urine samples; pre-visit 2: urine and blood samples, rowing maximal test familiarization) (Figure 1). Afterward, participants were assigned to a dietary group at random sampling, either an alkalizing (ALK, n = 12) or an acidizing (ACI, n = 12) diet. A daily check was conducted between our nutritionist and participants to ensure that they were adhering to the nutritional guidelines required by their respective groups. During weeks 2 and 4, participants were instructed to drink 1.5 L to 2 L of either bicarbonate-rich water (STY) or placebo water (PLA) in a cross-over, double-blind, and randomized design as part of their daily hydration. At the end of weeks 2 and 4, a supra-maximal test consisting of a 1 min rowing Wingate Test (1-AO) was completed, with blood biomarkers measured under each condition (STY or PLA).
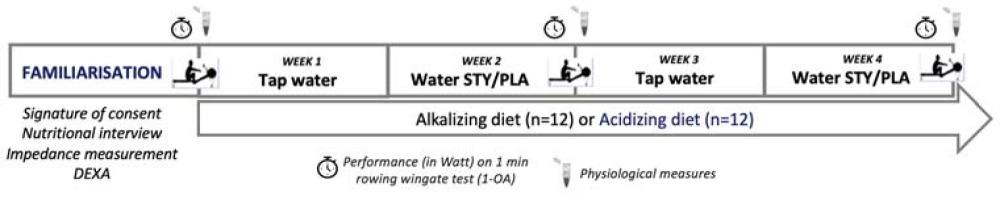
Figure 1
Schematic overview of the study design. During weeks 2 and 4, participants consumed bicarbonate-rich water (STY) or placebo water (PLA) in a cross-over design at a rate of 1.5 to 2 L per day, with habitual hydration resumed during weeks 1 and 3. At the end of each week, a supra-maximal test consisting of a 1-min rowing Wingate test (1-AO) was completed with physiological biomarkers being measured meanwhile. Participants followed also an acidizing (n = 12) or an alkalizing (n = 12) diet for four weeks.
Experimental Water
In both weeks 2 and 4, in a double-blind and randomized fashion, participants were instructed to drink 1.5 to 2 L per day of either bicarbonate-rich or placebo water (Table 1) during the first six days of the week. Four bottles of 500 mL were provided daily to each participant. St Yorre water (STY) (Vichy St Yorre, Sources Alma, La Ferrière Bochard, France) is a naturally carbonated water rich in minerals (4774 mg/L containing 4368 mg of HCO3- ions). The low-bicarbonate water, as placebo (PLA), was provided to participants in identical bottles to STY, but contained regular carbonated water with considerably lower mineral content (Table 1). Habitual hydration was resumed during weeks 1 and 3.
Table 1
Composition of St Yorre water and Placebo water.
Dietary Group
Next to a nutritional interview, participants were assigned at random sampling alkalizing or acidizing diet. To maintain single-blind dietary strategies during the protocol, the diet was presented as a vegetarian- or meat-prone diet. Each participant was given detailed written nutritional guidelines and meal plans for the duration of the study. The ALK diet was based on a vegetarian-prone diet (Caciano et al., 2015; Limmer et al., 2018). Participants were required to consume a minimum of three portions of vegetables and four portions of fruit daily. Dairy products (including yogurt, fresh cheese, curd, and milk) and potatoes were permitted.
Conversely, participants were prohibited from consuming meat, fish, and cheese besides fresh cheese and non-whole meal starchy foods. In contrast, the ACI diet was based on a meat-prone diet (Caciano et al., 2015; Limmer et al., 2018). Participants consumed three portions of animal protein (meat, fish, or eggs), three portions of starch, and three portions of dairy including at least one portion of cheese and were required to avoid consuming any bean, vegetable or fruit.
During the entire protocol, a trained dietician monitored dietary intake via a written food diary and accompanying photographs of meals to ensure that participants followed the recommandations. Moreover, the Potential Renal Acid Load (PRAL) (Remer and Manz, 1995) was calculated to verify the characteristic of either the alkalizing or the acidizing diet. The goal for the alkaline-trending diet group (ALK) was to achieve a dietary PRAL ≤ −1 mEq /d, and for the acid-trending group (ACI), a PRAL ≥ 15 mEq/d (Peter et al., 2010).
Data Collection
Urine Analysis
During the 6th day of weeks 2 and 4, participants completed a 24-h urine collection. Urinary pH was assessed using a pH probe (914, Metrohm, Switzerland).
On day 7 of each week, participants reported to the laboratory having consumed the same dietary intake for the 24 h before each visit.
1-AO Test
In the week before the commencement of the study, participants completed a 1-AO which served as a familiarization for the upcoming trials. Participants performed a 10-min, self-paced warm-up (replicated between trials) followed by a maximal 1-AO on a rowing machine (PM5, Concept2, USA) with instructions to complete an “all-out” effort and not to be concerned about pacing strategies. Performance was assessed as maximum (Pmax) and average (Pmean) power output in absolute (W) or relative to body weight (W/kg) values. Perception of effort (RPE) and the fatigue index in the last 10 s of the test (IDF 50–60 = [Pmean 50–60 − Pmax] / Pmax) were also determined.
Blood Acid-Base Status
Blood biomarkers, including blood lactate concentration ([La]), blood HCO3- concentration ([HCO3-]), and blood pH, were measured to determine acid-base regulation weekly (day 7) via earlobe-prick samples (20 μL) after the warm-up, immediately after exercise (+0'), then 3 (+3'), 5 (+5'), and 8 (+8') minutes (passive recovery) following the 1-AO. Samples were analyzed via blood gas and electrolyte analyzer (i-STAT, Abbott, USA).
Statistical Analyses
Statistical analyses were performed with RStudio software (version 3.5.0, Inc. Boston, MA, USA). To assess the effect of water (STY vs. PLA) on acid-base balance and performance, we applied a linear mixed model on the whole population with water as a fixed effect. The model was adjusted for the dietary group as the dietary group was also included as a fixed effect.
After that, two mixed models were created, one for each dietary group, to evaluate to what extent diet modulated the effect of water intake. Within each model, water intake was maintained as the fixed effect, and the random effect was the subject (STYALK vs. PLAALK and STYACI vs. PLAACI). The variables studied were biomarkers of acid-base balance and performance. The characterization of the diet as either acidizing or alkalizing was assessed against the PRAL of the diet. Comparison of PRAL was assessed with Student’s t tests either for paired data (STYACI vs. PLAACI and STYALK vs. PLAALK) or independent groups (ACI vs. ALK). The normality of data was checked via the Shapiro-Wilk test before using this parametric test.
The results are presented as means ± standard deviations. The coefficient of the model represents the mean difference when placed in a condition relative to the reference condition in the unit of measurement of the variable studied. A difference was considered significant at p < 0.05. Cohen's d was calculated, enabling us to categorize the magnitude of the error of estimation as large (0.8), moderate (0.5–0.8), or small (0.5) and to facilitate a more in-depth discussion of our results (Rosenthal and al., 1994).
Results
Water Consumption and Bicarbonate Intake
The mean daily volume of water ingestion was 1919 ± 65 mL and 1842 ± 34 mL, with a corresponding bicarbonate ion content of 8381 ± 539 mg and 564 ± 39 mg for STY and PLA, respectively. The resultant relative sodium bicarbonate intake (given that 1 g of sodium bicarbonate contains 726 mg of bicarbonate) was calculated to be 146 ± 22 mg/kg/day and 10 ± 2 mg/kg/day for STY and PLA, respectively.
PRAL of Dietary Groups
The mean PRAL of ACI and ALK groups was significantly different (ACI: 49 ± 13 mEq/d vs. ALK: −13 ± 14 mEq/d, p < 0.001). The mean PRAL of the ALK group was within the fixed range for alkalizing diet (PRAL < 1 mEq/d) and was not different between STYALK and PLAALK conditions (STYALK: −12 ± 9 mEq/d PLAALK: −14 ± 18 mEq/d, p = 0.81).
The mean PRAL of the ACI group was within the fixed range for acidizing diet (PRAL > 15 mEq/d) and was not different between STYACI and PLAACI conditions (STYACI: 51 ± 9 mEq/d vs. PLAACI: 47 ± 15 mEq/d, p = 0.29).
Performance
There was no statistical difference in performance variables during the 1-minute supra-maximal test between STY and PLA water groups.
Absolute Pmax, absolute Pmean, relative Pmean (w/kg), Pmax, the fatigue index, and the RPE were not significantly different between the alkaline diet combined with placebo water and the alkaline diet combined with bicarbonate-rich water (d = −0.14, −0.09, −0.15, −0.05, −0.07, −0.27, respectively); and between the acidic diet associated with placebo water and the acidic diet associated with bicarbonate-rich water (d = −0.33, 0.14, −0.36, 0.18, −0.28, 0.14, −0.31, respectively).
Biomarkers of Acid-Base Balance
Urinary markers
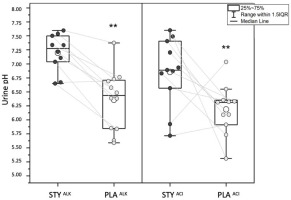
Urinary pH was significantly higher following the consumption of bicarbonate-rich water (STY) compared to placebo water (PLA) after 3 (STY: 7.06 ± 0.50 vs. PLA: 6.14 ± 0.46; p = 0.01; d = 1.92) and 6 (STY: 7.397 ± 0.3 vs. PLA: 7.387 ± 0.02, p = 0.01; d = 1.51) days of consumption.
Urinary pH measured following 24-hour urine collection on the 6th day of water consumption was significantly higher in STYALK than in PLAALK (STYALK: 7.20 ± 0.34 vs. PLAALK: 6.36 ± 0.54, p = 0.01; d = 1.93). It was also significantly higher in STYACI compared to PLAACI (STYACI: 6.86 ± 0.60 vs. PLAACI: 6.19 ± 0.45, p = 0.01; d = 1.28) (Figure 2).
Effects of Combined Bicarbonate-Rich Water and Diet
Blood lactate Concentration
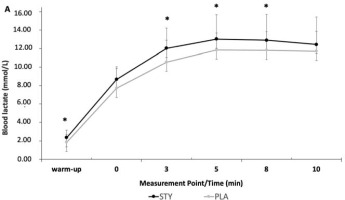
Blood lactate concentrations ([La]) were significantly higher in the STY compared to the PLA group during the warm-up and 3, 5, and 8 min after the supramaximal test. Peak blood lactate ([La]max) was significantly higher in the STY (13.4 ± 2.44 mmol/L) compared to the PLA group (12.45 ± 2.65 mmol/L; p = 0.035; d = 0.37) (Figure 3).
Figure 3
Blood lactate at the end of the 1-min supra-maximal test during passive recovery after consumption of bicarbonate-rich water (STY) or placebo water (PLA) for 1 week. * Indicates a value significantly different from the PLA condition (p < 0.05). Warm-up = measurement taken after the 10-min rowing warm-up and before the supra-maximal test; +0 = immediately after the supra-maximal test; +3, +5, +8 and +10: 3, 5, 8 and 10 minutes after the supra-maximal test. The data are presented as means ± standard deviations.
Blood Lactate
Peak Blood lactate [La] was significantly higher in the STYALK compared to the PLAALK group (p < 0.01; d = 0.51), as well as after the warm-up and 5 min after the 1-AO during passive recovery (p = 0.02; d = 0.88 and p = 0.04; d = 0.65). Regarding ACI, only the peak of [La] was significantly higher in the STYACI compared to the PLAACI group (p < 0.01).
Blood pH
Blood pH was significantly higher in the STY than the PLA group immediately following, 3, and 5 minutes after the supramaximal test (Figure 4).
Figure 4
Blood pH after the 1-min supra-maximal test during passive recovery from consumption of bicarbonate-rich water (STY) or placebo water (PLA) for 1. * indicates a significantly different value (p < 0.05) of PLA. Warm-up = measurement taken after the 10-min rowing warm-up and before the supra-maximal test; +0' = immediately after the supra-maximal test; +3, +5 and +8: 3, 5 and 8 min after the supra-maximal test. The data are presented as means ± standard deviations.
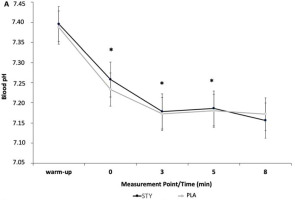
Blood pH was significantly different for the STYALK compared to the PLAALK group directly after 1-AO (+0') (p < 0.01, d = 0.79) and 5 min post-exercise (+5') (p = 0.02, d = 0.22). No significant difference was observed in blood pH in the STYACI compared to the PLAACI group.
Blood HCO3- Concentration
Blood bicarbonate (HCO3-) concentrations were not significantly different between STY and PLA groups at any timepoint before or after the 1-min supra-maximal test. Peak blood [HCO3-] was significantly different for the STYALK compared to the PLAALK group as well as after the warm-up (p = 0.02, d = 1.01) and directly after the 1-AO (+0') (p < 0.01, d = 0.58) (Figure 5). No significant difference was observed for blood [HCO3-] in the STYACI compared to the PLAACI group.
Figure 5
Blood bicarbonate concentration ([HCO3-]) at the end of the 1-min supra-maximal test during passive recovery from consumption of bicarbonate ion-rich water (STYALK) or placebo water (PLAALK) for 1 week in combination with an alkalizing diet. * Indicates a significantly different value (p < 0.05) of PLA. Warm-up = measurement taken after the 10-minute rowing warm-up and before the supra-maximal test; +0' = measurement taken immediately after the supra-maximal test; +3, +5 and +8: measurements taken 3, 5 and 8 min after the supra-maximal test. The data are presented as means ± standard deviations.
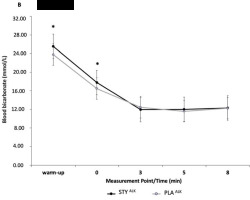
Peak blood [HCO3-] was significantly different for the STYALK group compared to the PLAALK group, as well as after the warm-up (p = 0.02, d = 1.08) and directly after the 1-AO (+0') (p < 0.01, d = 0.58) (Figure 5). No significant difference was observed for blood HCO3- concentration in the STYACI compared to the PLAACI group.
Discussion
This study was the first to investigate the influence of chronic bicarbonate supplementation via the consumption of water naturally rich in bicarbonate ions on the regulation of acid-base balance and anaerobic performance. Furthermore, to determine whether alkalizing or acidizing diets could modulate the effect of bicarbonate-rich water, we subdivided our population into two groups. We reported that the consumption of naturally carbonated bicarbonate ion-rich water induced a significant increase in urine pH compared to regular carbonated water (p < 0.05), irrespective of the diet. Furthermore, despite no water or the diet type effect on anaerobic performance, peak blood lactate concentration during recovery was elevated following bicarbonate-rich water consumption compared to regular carbonated water. Additionally, blood lactate concentration increased comparably in both dietary groups (p < 0.05) following bicarbonate-rich water consumption. Post-exercise blood pH was significantly increased with the consumption of bicarbonate-rich water. This effect was maintained following the consumption of an alkalizing diet, whereas it was not with the acidizing diet. Lastly, consuming bicarbonate-rich water alongside an alkalizing diet conferred an additional buffering effect on blood bicarbonate concentration after exercise (p < 0.05).
Consistent with the work of Heil et al. (2010), we observed a significant increase in 24-h urine pH after 3 (STY: 7.06 ± 0.50; PLA: 6.14 ± 0.46; p = 0.01) and 6 days (STY: 7.02 ± 0.51; PLA: 6.28 ± 0.49; p = 0.01) of consumption of mineral water rich in bicarbonate ions in comparison to PLA (p < 0.01). Heil et al. (2010) similarly evaluated the impact of alkaline water consumption on acid-base balance and hydration status. However, we reported a faster and more marked effect of bicarbonate ion-rich water consumption on urinary pH, which modified the circulating acid-base balance. Moreover, the alkalinizing effect of bicarbonate-rich water on the urine pH was further altered when combined with alkalizing and acidizing diets. The consumption of bicarbonate-rich water compensated for the acidizing diet-induced acid load on the acid-base balance.
Although no effect of water and the diet type was reported on anaerobic performance, we observed in STY, STYALK, and STYACI significantly higher values of peak blood lactate concentration after 1-AO compared to PLA, PLAALK, and PLAACI. We also observed in STY, and STYALK significantly higher values of blood lactate, and pH, after 1-AO compared to PLA and PLAALK. Furthermore, HCO3- concentration was augmented when bicarbonate-rich water consumption was combined with an alkalizing diet, characteristic of bicarbonate supplementation (Carr et al., 2011). Indeed, changes in these blood biomarkers, indicative of acid-base balance, have previously been reported following threee weeks of alkaline water intake (Chycki et al., 2018), following the consumption of bicarbonate-rich water during aerobic exercise (Rieux and Duvallet, 2000), and following four days of an alkaline diet (Limmer et al., 2018). Increased peak blood lactate may be explained by higher glycolytic rates, permitted by reduced inhibition of phosphofructokinase activity, an essential enzyme in the control of glycolysis (Robergs et al., 2004). Secondly, blood lactate concentration may be increased due to improved lactate transportation. Specifically, increased activity of transport proteins (membrane co-transporters MCT1 and MCT4) may facilitate the release of muscle lactate into the extracellular environment. The presence of bicarbonate ions, therefore, allows for the removal of extracellular H+, creating the necessary concentration gradient between the intra- and extracellular media, leading to the efflux of H+ and lactate from muscle during exercise (Robergs et al., 2004). An increase in muscle-released lactate concentrations has been previously observed, suggesting that elevated alkalosis accelerates lactate transport (Maughan et al., 2018). The variations in post-exercise blood pH, lactatemia, and HCO3- concentration in STYALK support the hypothesis of an additive buffering effect of the dietary (ALK) and water (STY) strategies, but not sufficient to cause ergogenic effects during the present exercise modality.
One of our initial hypotheses was that the consumption of bicarbonate-rich water could compensate for the effects of an increased acid load induced by the consumption of an acidizing diet on the acid-base balance. Urinary pH in STYACI was significantly higher than PLAACI (STYACI: 6.86 ± 0.60 vs. PLAACI: 6.19 ± 0.45; p = 0.01), underlining the significative effect of drinking bicarbonate-rich water on urine alkalinization. Although we did not find significant effects on blood biomarkers, the consumption of mineral water rich in HCO3- ions appears to reduce the acid load induced by the acidizing diet by regulating the acid-base balance.
In contrast to previously reported literature, the strategies implemented here did not influence performance with an alkalinizing diet (Limmer et al., 2018), chronic sodium bicarbonate intake (McNaughton and Thompson, 2001) or acute intake of bicarbonate-rich water (Rieux and Duvallet, 2000). In the study by McNaughton and Thompson (2001), which used a similar experimental design to ours, albeit with reduced ecological validity, bicarbonate supplementation in a capsule form resulted in improved anaerobic performance. Most notably, the quantity of bicarbonate administered was 3 to 4 times higher than in our study. Practically, achieving comparable levels of bicarbonate intake via bicarbonate-rich water ingestion would not be possible as participants would be required to drink 6 to 7 L of water daily. Additionally, using the same experimental water as STY in our study, Rieux and Duvallet (2000) reported reduced muscle fatigability at the end of the endurance test. Two major points differentiate this study from ours, the timing of intake and the nature of the test (single vs. repeated). Indeed, in the study by Rieux and Duvallet (2000) water was not ingested chronically but only on the day of testing, starting 2 h before the first exercise and lasting until the last test. The second difference lies in the test, either single exercise as in our study or repeated exercise. Data from the Rieux and Duvallet’s (2000) study suggest that drinking bicarbonate-rich water throughout the duration of repeated exercise might be of interest. Recent findings confirm that repeatedly ingesting a small amount of sodium bicarbonate improves repeated all-out performance when ingestion is spread throughout exercise (Dalle et., 2019).
More recently, Hadzic et al. (2019) reported large inconsistencies in the sodium bicarbonate supplementation literature with artificial dietary supplements with a higher dose of bicarbonate compared to this study, showing that effects on performance could differ even when the same exercise and supplementation protocols were applied (Saunders et al., 2014). Saunders et al. (2014) did not show any relationship between the magnitude of change in circulating biomarkers related to acid-base balance, such as bicarbonate and pH, and subsequent enhancement in performance of HI exercise of similar duration as in the present study. Also, as stated in the study of Thomas et al. (2021), there is considerable inter-individual variability in humans in the assimilation of an exogenous alkalinizing intake and its buffering effect. This might have contributed to the fact that we did not find any effect on performance despite the change in acid-base balance biomarkers.
Water naturally rich in bicarbonate ions can be utilized to supply the necessary bicarbonate ions for optimizing buffering capacity. Our study indicates that the consumption of bicarbonate-rich water over several days positively modifies acid-base balance. In practical terms, a daily intake of 1.5 to 2 l of bicarbonate-rich water as part of a hydration plan could gradually enhance buffering capacity in the days leading up to competition. It is recommended to initiate this strategy at least three days before the competition and distribute water intake throughout the day, such as 0.5 l in the morning, 0.5 l around noon (between meals), in the afternoon, and possibly in the evening. This approach could be particularly beneficial for athletes aiming to avoid excessive supplement consumption or who experience gastrointestinal issues (McNaughton et al., 2016 ). As highlighted in our study, an alkaline diet enhances the beneficial effect of bicarbonate-rich water on acid-base balance. Just like with hydration, athletes can optimize their diet by predominantly including fruits and vegetables in an alkaline-based diet. However, it is strongly recommended to test these nutritional strategies for each athlete during a training period or a non-competitive phase to prevent discomfort associated with dietary modifications. While this mode of consumption did not improve anaerobic performance, future research can explore whether the combination of chronic and acute intake of bicarbonate-rich water could enhance performance. Further studies could investigate the effects of these nutritional strategies, particularly in non-weight-bearing sports, among non-recreational athletes (Froio de Araujo Dias et al., 2015), and in the context of repeated exercise, especially when bicarbonate-rich water is consumed throughout the exercise duration. Future studies should take into account sodium supplementation, which could also have an impact on anaerobic performance (Durkalec-Michalski et al., 2023). Finally, one potential limitation of this study was the use of a basic randomization method, based on random division without taking into account the pre-visit outcomes. Indeed, participants were not matched based upon the primary outcomes of the previsit, for instance, the familiarization performance test, before being randomly assigned to one of the two dietary groups. Finally, considering that participants were recreationally trained men, we may hypothesize that the nutritional strategy implemented in our study could have enhanced effects on athletes with a higher level of performance. Indeed, following a high-intensity exercise, metabolic acidosis is more pronounced in elite athletes compared to athletes at the regional level (Hanon et al., 2011). Consequently, supplementing with bicarbonate-rich water along with an alkalizing diet could optimize the buffering capacity of more trained athletes.
Conclusions
In conclusion, this study was the first to investigate the influence of chronic consumption of bicarbonate-rich water on the regulation of acid-base balance and anaerobic performance and its combined effects with an alkalizing or an acidizing diet. Despite the implementation of these nutritional strategies, bicarbonate-rich water intake did not affect anaerobic performance, regardless of dietary intake. While we did not measure blood HCO3- concentration and pH before the warm-up, we showed that the chronic consumption of bicarbonate-rich water significantly impacted resting urinary pH irrespective of alkalizing or acidizing dietary intake. Furthermore, we observed significantly greater post-exercise peak blood lactate in response to bicarbonate-rich water consumption compared to the placebo condition. In addition, blood pH and bicarbonate concentration were affected by both bicarbonate-rich water and alkalinizing diet during the recovery period compared to the placebo condition. Our results suggest that consuming mineral water rich in HCO3- ions potentiates an alkalizing diet's beneficial effects on the biomarkers reflecting acid-base balance during a 1-AO. Finally, consuming bicarbonate-rich water reduces the acid load induced by an acidizing diet.